|
|
Postdoctoral Research at Caltech
My primary Caltech research project aimed at detecting and
characterizing a molecule known as HOONO. This elusive isomer of nitric acid and
suspected intermediate of the OH + NO2 + M association
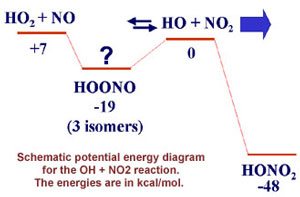 reaction
is calculated to be bound by some 19 kcal/mol. HOONO has been
observed in solid Argon matrices, and there was a compelling
evidence for its transient existence in aqueous solutions. However,
no one has ever seen it in the gas-phase, and not for the lack
of trying! Because of its small binding energy, HOONO should
readily decompose back into OH and NO2 upon 2v1
OH stretch excitation providing one with a very sensitive and
potentially background-free way of detecting it. Indeed, Paul
Wennberg and I succeeded [37] in observing the first photodissociation gas-phase spectrum
of HOONO and estimated its yield in the OH + NO2
reaction (5%). From an experimental kinetics perspective, the
successful observation of HOONO by photodissociation spectroscopy
under ambient temperature conditions opens the door to many
exciting studies of reactions involving weakly-bound adducts
such as, for example, ROONO and RO2-H2O
(R = organic radical). Later on, Brian Bean, Andrew Mollner,
Mitchio
Okumura, Stanley
Sander, and myself detected [39] this molecule using cavity ring-down
spectroscopy and provided a more qualitative measurement of
the yield as a function of temperature and pressure. Still later on, Julie Fry, John Crounse,
Coleen Roehl, and Paul
Wennberg measured thermal lifetime of HOONO [41]. reaction
is calculated to be bound by some 19 kcal/mol. HOONO has been
observed in solid Argon matrices, and there was a compelling
evidence for its transient existence in aqueous solutions. However,
no one has ever seen it in the gas-phase, and not for the lack
of trying! Because of its small binding energy, HOONO should
readily decompose back into OH and NO2 upon 2v1
OH stretch excitation providing one with a very sensitive and
potentially background-free way of detecting it. Indeed, Paul
Wennberg and I succeeded [37] in observing the first photodissociation gas-phase spectrum
of HOONO and estimated its yield in the OH + NO2
reaction (5%). From an experimental kinetics perspective, the
successful observation of HOONO by photodissociation spectroscopy
under ambient temperature conditions opens the door to many
exciting studies of reactions involving weakly-bound adducts
such as, for example, ROONO and RO2-H2O
(R = organic radical). Later on, Brian Bean, Andrew Mollner,
Mitchio
Okumura, Stanley
Sander, and myself detected [39] this molecule using cavity ring-down
spectroscopy and provided a more qualitative measurement of
the yield as a function of temperature and pressure. Still later on, Julie Fry, John Crounse,
Coleen Roehl, and Paul
Wennberg measured thermal lifetime of HOONO [41].
Top
Postdoctoral Research at JILA
I was responsible for three research projects in Prof.
Nesbitt's group at JILA
(University of Colorado at
Boulder and NIST).
 The first one was concerned with the state-resolved dynamics
of chemical reactions involving highly reactive fluorine atoms
studied in crossed supersonic jets using high-resolution infrared
direct absorption spectroscopy. The most significant example
from that work was the reaction F + H2 -> HF(v, J)
+ H and its isotopic variant, F + HD. Using a combination of
an ultrasensitive detection of HF, single-collision environment
of the crossed jets, and a high quantum state selectivity we
succeeded in measuring the full nascent HF(v,J) ro-vibrational
state distribution for the first time [27]. In addition, our study
provided the first definitive observation of non-adiabatic
(Born-Oppenheimer forbidden) reaction between the spin-orbit
excited fluorine atom and H2, predicted by many theoreticians
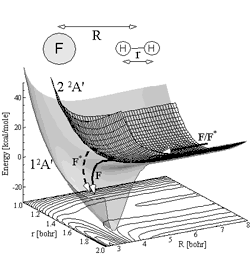
but never observed in an experiment [29,30]. Figure on the right shows
a schematic diagram of the relevant potential energy surfaces
involved in this process. As a step towards understanding the
dynamics of atom-polyatom processes we also undertook a study
of an atom-pentatom reaction F + CH4 -> HF(v, J)
+ CH3 at a similar level of quantum precision and
detail [31,33].
The first one was concerned with the state-resolved dynamics
of chemical reactions involving highly reactive fluorine atoms
studied in crossed supersonic jets using high-resolution infrared
direct absorption spectroscopy. The most significant example
from that work was the reaction F + H2 -> HF(v, J)
+ H and its isotopic variant, F + HD. Using a combination of
an ultrasensitive detection of HF, single-collision environment
of the crossed jets, and a high quantum state selectivity we
succeeded in measuring the full nascent HF(v,J) ro-vibrational
state distribution for the first time [27]. In addition, our study
provided the first definitive observation of non-adiabatic
(Born-Oppenheimer forbidden) reaction between the spin-orbit
excited fluorine atom and H2, predicted by many theoreticians
but never observed in an experiment [29,30]. Figure on the right shows
a schematic diagram of the relevant potential energy surfaces
involved in this process. As a step towards understanding the
dynamics of atom-polyatom processes we also undertook a study
of an atom-pentatom reaction F + CH4 -> HF(v, J)
+ CH3 at a similar level of quantum precision and
detail [31,33].
This figure shows the relevant potential energy surfaces involved in the F + H2
reaction. Non-adiabatic reaction starts on the upper (repulsive)
potential energy surface.
The second project
was related to the kinetics and dynamics of reactions involving
hydroxyl radical (OH) aimed to improve our understanding of
the intricate chain of radical-driven chemical processes occurring
in the Earth's atmosphere. We undertook a detailed study of
the OH/HO2/O3 chain reaction kinetics
in a broad range of temperatures (190-320 K) using flash-photolysis
and direct absorption infrared spectroscopy [32]. This reaction cycle
is responsible for up to 50% of the natural ozone decomposition
at mid-latitudes and accurate rate constants we obtained in
that study are now used in the latest NASA
compilation of kinetic parameters for atmospheric modeling.
The third project
was centered around the dynamics of quantum state-resolved photodissociation
of water and water-based Van der Waals complexes. In this challenging
three-laser experiment, either free or bound water molecules
are prepared in a specific quantum state via direct overtone
pumping, and subsequently photodissociated with ultraviolet
radiation. The quantum state distribution of the resulting OH
fragment is thoroughly interrogated by laser induced fluorescence [38].
This study have resulted in an unprecedented amount of detailed
information on vibrationally mediated dissociation of H2O,
Ar-H2O, and water dimer. Observation of the first
rotationally resolved (H2O)2 overtone
transition represents the most exciting finding of that work [42].
Top
Graduate Research at the University of Basel
During my graduate career at the Institute
of Physical Chemistry in Basel (advised by Evan
Bieske, Otto
Dopfer, and John
Maier ), I was involved in studies of the structure, reactivity
and dynamics of weakly-bound ionic complexes and clusters by
means of ion photodissociation spectroscopy [link to papers on ion complexes]. In this method,
the ions of interest are mass-selected with a quadrupole mass
spectrometer and injected into an ion trap where they are photofragmented
via their electronic or infrared transitions. The charged photofragments
are separated from the parent ions with another mass-spectrometer
and detected in essentially background-free fashion. With this
form of action spectroscopy we were able to obtain unique spectroscopic,
dynamical and structural information about a number of ionic
complexes for the first time. Systematic studies of proton-bound
complexes of the type A--HB+ (A = He, Ne, Ar,
H2; B = CO, N2, H2O, NH3,
etc.) led to an improved understanding of bonding mechanisms
in these intriguing systems. We established a number of useful
correlations between the proton bridge stability and the proton
affinities of individual bases comprising the complex. More
specifically, we found that such proton-bound complexes favor
a relatively rigid linear A--H-B arrangement, with the strongest
hydrogen bonds formed between bases with similar proton affinities.
For example, binding energy of Ar-HN2+
complex determined by our group is as large as 2781.5+/-1.5
cm-1, which, incidentally, represents one of the
most precise reported measurements of bond strength in a weakly-bound
system. Larger disparity in the proton affinities leads to progressively
weaker H-bridging and floppier structures up to the extreme
case of He-NH4+ complex, which is characterized
by essentially free rotation of the NH4+
core ion and a binding energy of less than 100 cm-1.
Studies of larger proton-bound complexes (An--HB+)
successfully addressed the questions of cluster growth, differential
solvation, and connection between microscopic and bulk properties
of solvated ions. For example, we found several easily recognizable
spectroscopic signatures of shell growth in Arn--HCO+,
Arn--NH4+ and similar systems.
Top
Undergraduate Research at the Novosibirsk
State University
PI's undergraduate research in the Institute of
Chemical Kinetics and Combustion in Novosibirsk, Russia,
was concerned with radiationless transitions in nitrogen dioxide
induced by a magnetic field [1] and with photochemistry of electronically
excited NO2 [4]. The most interesting finding of that
work was the reaction of electronically excited NO2
with the ground state NO2, which, in addition to
the standard excitation quenching route, produced significant
yields of NO3 radical [4]. The work was done in the laboratory
of Prof. Nikolai
Bazhin and it was supervised by Dr. Vladimir
Makarov
Top
NO2 high-resolution absorption
cross-section data
Absolute absorption cross-sections of NO2 measured
with a high-resolution Fourier transform spectrometer at Kitt
Peak National Solar Observatory
Work Info: The spectra were recorded [40] in synthetic
air at a 0.060 cm-1 resolution in the 415-525 nm
range with a high-precision Fourier-transform
spectrometer. The measurements were conducted for a range
of pressures (1-760 Torr) and temperatures (220-298 K) that
are representative of typical tropospheric and stratospheric
conditions. Maximum uncertainty for the reported absolute absorption
cross sections is 7% (two sigma), which is primarily limited
by the light source drifts and by uncertainties in NO2
concentrations. Note that the error is likely to be largest
at the edges of the spectral range studied here; 5% is probably
a better conservative estimate for the middle of the investigated
range. The wavelength (referred to vacuum) accuracy is 0.011
cm-1 (2.8x10-4 nm at 500 nm) and precision
is 0.0022 cm-1 throughout the investigated wavelength
range.
File Info: Files are saved as individual
columns of ASCII text data with 4 significant digits of precision.
Absorption cross section units are cm2/molecule;
natural logarithm base. Vacuum wavenumber scale in cm-1
is saved in a separate file with 10 significant digits of precision
(download).
Alternatively, the vacuum wavenumber scale can be reconstructed
from the following end values: Start = 18000.023179375 cm-1
; End = 24499.973540343 cm-1. All files are compressed
using "ZIP" standard.
|
P (Torr) |
T (K) |
[NO2]
(#/cm3) |
[N2O4]
(#/cm3) |
|
|
596.10 |
298.6 |
1.33E+15 |
4.82E+11 |
download |
| 302.20 |
298.8 |
1.33E+15 |
4.76E+11 |
download |
| 151.00 |
298.9 |
1.34E+15 |
4.77E+11 |
download |
| 75.45 |
298.6 |
1.32E+15 |
4.75E+11 |
download |
| 1.99 |
298.4 |
3.01E+15 |
2.51E+12 |
download |
|
0.49 |
298.2 |
7.51E+14 |
1.58E+11 |
download |
|
760.50 |
273.2 |
2.07E+15 |
9.20E+12 |
download |
| 421.50 |
273.0 |
2.01E+15 |
8.83E+12 |
download |
| 151.20 |
272.9 |
1.77E+15 |
6.93E+12 |
download |
|
1.24 |
272.6 |
2.05E+15 |
9.57E+12 |
download |
|
309.50 |
249.6 |
1.53E+15 |
4.98E+13 |
download |
| 309.00 |
249.5 |
1.53E+15 |
5.05E+13 |
download |
|
1.85 |
249.2 |
2.97E+15 |
1.97E+14 |
download |
|
233.80 |
230.8 |
1.67E+15 |
5.18E+14 |
download |
| 1.67 |
229.2 |
1.81E+15 |
7.48E+14 |
download |
| 211.30 |
228.7 |
1.41E+15 |
4.89E+14 |
download |
|
117.84 |
226.6 |
1.37E+15 |
6.01E+14 |
download |
|
300.50 |
214.7 |
1.58E+15 |
4.07E+15 |
download |
| 155.30 |
214.7 |
1.61E+15 |
4.24E+15 |
download |
| 41.27 |
214.0 |
1.61E+15 |
4.66E+15 |
download |
| 5.07 |
215.1 |
1.72E+15 |
4.49E+15 |
download |
You can also download I2 calibration scans 1
and 2, and a combined
I2 / NO2 calibration scan.
Top
|


